Navy Medical Department Guide to Malaria Prevention and Control
Appendix 3: Laboratory Diagnostic Techniques
Department of the Navy
Bureau of Medicine and Surgery
Introduction. Despite development of serological techniques, conclusive
diagnosis of malaria continues to be made through microscopic examination of peripheral
blood smears. This is the only method that can differentiate among the four species of
plasmodia that cause human malaria.
Thick Smears. Red blood cells are hemolyzed in thick smears; leukocytes and any
malaria parasites present are the detectable elements. The hemolysis and slow drying that
occur in thick smear preparation cause distortion of plasmodia morphology, making
differentiation of species difficult. Thick smears are used to detect infection, and
estimate parasite concentration.
Thin Smears. Thin smears are fixed with methanol, preventing hemolysis. Red
blood cells are intact, and any Plasmodia present are less likely to be distorted, and
remain within erythrocytes. Identification of specific species is usually done using thin
smears after detection of parasites on the thick smears.
Navy Environmental & Preventive Medicine Units offer training classes on
preparation of thick and thin smears and microscopic examination for diagnosis of malaria.
This appendix summarizes thick and thin blood smear preparation for field reference.
Drawing Blood
- Anytime malaria is suspected.
- Repeat if smears are negative.
- Maximum frequency: once per hour.
Obtaining Blood
- Fresh blood is required from either fingerstick or venous phlebotomy
- Follow universal precautions (gloves, hand washing, proper handling and disposal of
sharp instruments and other materials contaminated with blood)
- Fingerstick Method:
- Clean end of finger with disinfectant solution.
- Wipe fingertip with sterile material (remove remaining disinfectant that may interfere
with diagnostic process).
- Pierce fingertip with sterile lancet.
- Allow blood to flow freely, do not squeeze finger.
- For venous blood obtained in a vacutainer, use a pipette to apply a drop of blood to
slide(s) for thick and thin smears
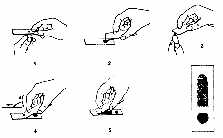
Slide Preparation (see Appendix Figure 3-1)
- Thick Smear
- Wipe away first drop of blood at fingerstick site. Then touch a clean microscope slide
near one end to the next blood drop that forms.
- Spread drop of blood with corner of another slide to make an area about 1 cm in
diameter.
- This is the thick smear. Correct thickness is attained when newsprint is barely legible
through the smear.
- Thin Smear
- Touch a new drop of blood (smaller than the first) with the edge of a clean slide.
- Bring the edge of the slide with the new drop of blood to the surface of the first
slide. Place it at the far end, and wait until the blood spreads along the whole edge.
- Holding the slide at an angle of 450, push it forward with a rapid, gentle movement.
- For preparation of separate slides for thick and thin smears, use a second slide in step
4.
- Dry the smears. Air dry, allowing 10 minutes for the thin smear and 30 minutes for the
thick smear.
- Mark slide with patient identification and date and time of collection. This can be done
using a pencil on the thin smear after it has dried.
- Fixing Thin Smears
- After drying, only thin smears are fixed. Fixing is done using methanol in one of two
ways:
- Dip thin smear into methanol for 5 seconds.
- Dab thin smear with methanol-soaked cotton ball.
- Do not fix the thick smear. Even exposure of the thick smear to methanol fumes will
prevent hemolysis and make it unreadable. If using the one slide method, prevent exposure
of the thick smear to methanol or methanol fumes by carefully dipping or dabbing the
slide, and gently blowing the fumes away from the thick smear area.
Staining Slides
Giemsa stain is available in the military supply system, and this staining method is
presented. Preparation of Giemsa staining solution is done with buffered water and Giemsa
concentrate. Do not shake the Giemsa concentrate as this will cause suspension of
particulate matter in the stain resulting in artifacts on final slides. Formation of
artifacts renders slides difficult to interpret.
Preparation of Giemsa staining solution
- Prepare buffered water solution, pH 7.2:
- Mix capful of buffering salts into 1000 ml of distilled water.
- Check pH. Titrate with sodium hydroxide (NaOH) solution until pH is 7.2.
- Prepare Giemsa staining solution by mixing:
- 1 part unshaken Giemsa stain concentrate.
- 9 parts buffered water.
Appendix Figure 3-1. Thick and Thin Blood Smear Preparation.
Slide Staining with prepared Giemsa solution
- Place slides flat in a staining rack or other suitable surface.
- Cover with 1-2 ml of Giemsa solution.
- Let stand for 10 minutes.
- Gently rinse by "floating" excess stain off slide with buffered water; be
careful not to wash the blood smear away.
- Rinse until no more stain is seen in solution.
- Dry smear side down, making sure that smear does not touch the slide rack or other
surface used for drying.
Slide Preparation Pointers
- Clean microscope slides before use. Blood will spread cleanly, stain will adhere
properly, and no artifacts will impede diagnosis.
- Do not fix slides with a heat source. If overexposed to heat, parasites are destroyed,
and cannot be seen microscopically.
- Parasites stain best at pH of 7.2. Check stain pH for optimal staining.
- Filter the Giemsa stain. Removal of particles and residue will make slides much easier
to interpret.
Microscopic Examination of Thick and Thin Blood Smears
Training and experience is essential for accurate reading. Slides should be examined
for at least 20 minutes before being judged to be free of malaria parasites. Parasites are
often not readily apparent, and quick visual scans are insufficient for diagnostic
purposes. Appendix Table 3-1 shows selected microscopic characteristics of human malaria
species.
Part of the diagnostic process is estimation of the extent of infection. Two methods
are presented to estimate the parasite concentration or parasitemia. One requires the use
of thick smears, and is called the Absolute Numbers Method. The other requires the
use of thin smears, and is called the Percent Method.
Absolute Numbers Method (thick smear)
This method is based on the assumption that 8000 leukocytes (white blood cells) are
found in a ml of blood. By counting the number of parasites seen in same visual fields
needed to count either 200 or 500 leukocytes, the parasite concentration per ml can be
estimated. Perform the following steps to estimate parasite concentration:
- Examine the equivalent of 0.25 ml of blood (100 visual fields using a 7X ocular lens and
a 100X oil-immersion objective lens) to determine if an infection exists.
- In a systematic manner of scanning visual fields, identify 200 leukocytes, while
counting the number of malarial parasites in those same visual fields.
- If after 200 leukocytes have been identified and less than 9 malarial parasites have
been counted, continue the process until 500 leukocytes have been identified.
- If after 200 leukocytes have been identified and 10 or more parasites have been counted,
record the number of parasites counted per 200 leukocytes.
- Once 500 leukocytes have been identified, record the number of parasites counted.
- Convert the parasite count per leukocytes identified into parasite concentration per ml
with one of the following formulas:
Number of parasites x 8000 = parasites per ml
Number of leukocytes counted
or
if 200 leukocytes were counted:
Number of parasites counted x 40 = parasites per ml
or
if 500 leukocytes were counted:
Number of parasites counted x 16 = parasites per ml
- All parasite species and forms are tabulated together. This includes both sexual
(gametocytes) and asexual (trophozoites, merozoites) forms.
Appendix Table 3-1. Selected microscopic characteristics
| Species |
Stages found in circulating Blood |
Appearance of Red Blood Cells
Size Stippling |
|
| Plasmodium falciparum |
Trophozoites
Gametocytes |
Normal |
Maurer's dots or clefts infrequently seen |
| Plasmodium vivax |
All: Schizonts Trophozoites
Gametocytes |
Enlarged, Maximum size may be 1.5-2 times normal |
Schuffner's dots may be present |
| Plasmodium ovale |
All: Schizonts Trophozoites
Gametocytes |
Enlarged, Maximum size may be 1.25-1.5 times normal |
Schuffner's dots may be present |
| Plasmodium malariae |
All: Schizonts Trophozoites
Gametocytes |
Normal |
Ziemann's dots rarely seen |
Appendix Table 3-1. Selected microscopic characteristics (con't)
| Appearance of Parasites |
|
|
| Cytoplasm |
Pigment |
Number of merozoites |
| Double dots in rings common, rings small and delicate |
Black
Coarse and conspicuous in gametocytes |
6-32
Avg. = 10-24 |
| Ameboid trophs light blue, has irregular "spread out" appearance in troph
stage |
Golden-brown, inconspicuous |
12-24
Avg. = 16 |
| Rounded, compact trophs, dark to medium blue, usually dense; Chromatin is large |
Dark brown, conspicuous |
6-14
Avg. = 8 |
| Rounded, compact trophs, dark blue with dense cytoplasm; band form trophs
occasionally seen |
Dark brown, coarse, conspicuous |
6-12
Avg. = 8
"Rosette" occasionally seen |
Percent Method (thin smear).
This method estimates the percentage of red blood cells infected with malarial
parasites. It is based on the number of red blood cells found parasitized on a thin smear,
and is executed in the following manner:
- Locate an area in the thin smear where red blood cells are close together but not
touching.
- Scan in a systematic method (use the microscope stage control to scan one
"row" at a time).
- Count the total number of red blood cells in each row. At the same time, tabulate the
number of red blood cells parasitized.
- Count a total of 300-500 red blood cells.
- Divide the number parasitized by the total number counted and multiply the result by 100
to obtain a percentage estimate of red blood cells parasitized:
Red Blood Cells parasitized X 100 = Percent of Red Blood
Total Red Blood Cells counted Cells parasitized
- If occasional parasites are seen when scanning the smear, but none are identified during
the process of counting 300-500 red blood cells, a percentage value of less than 1% of red
blood cells parasitized is assigned.
- An estimate of less than 1% of red blood cells parasitized does not need to be refined,
since no clinical predictive value is gained. It is values of 2-3% or above that are of
clinical concern.
Future Diagnostic Techniques
New, easy to perform serum serology techniques for malaria diagnosis are awaiting FDA
approval. Two of these methods (ParaSightRF, Becton Dickinson, Sparks, MD; ICT
Malaria P.fR, ICT Diagnostics, Sydney, Australia) are designed for easy
performance in field situations, and require no laboratory experience and little training.
Both tools detect P. falciparum serologically using enzyme linked immunosorbent assay
(ELISA), with results in 10 minutes. Clinical trials show these methods detect P.
falciparum infections at parasite blood concentration of greater than 40 parasites per
microliter (>40 parasites/ml).
Advertise on this Site

